One of our MMRF Research Fellows, Dr. Holly Lee of the Arnie Charbonneau Cancer Institute, University of Calgary, Canada, has published an insightful review article about bispecific antibodies in multiple myeloma. We asked her to share her background and help us describe the content of her article.
I am a clinician-scientist in-training. I completed my clinical hematology fellowship in 2021 and have since been enrolled in the Clinician Investigator Program at University of Calgary pursuing a PhD under the supervision of Dr Nizar Bahlis. T-cell redirecting immunotherapies are rapidly transforming how we treat patients with multiple myeloma (MM) in the clinic. However, some patients do not respond to these immunotherapies and others relapse after initial response. My research interests are in understanding the biological mechanisms underlying MM resistance to these novel agents and in developing improved therapeutics that can overcome these barriers.
Receiving MMRF research grant support is invaluable for my career development as an early-career investigator. The funding will help expand our project on investigating tumor intrinsic and extrinsic pathways that drive MM resistance to bispecific T-cell engagers and chimeric antigen receptor T-cell therapies. The insights gained from this research have the potential to optimize immunotherapy selection and sequencing in clinical settings and guide the development of next generation T-cell immunotherapies – areas that will be the primary focus of my research career.
_____________________________________________________________________________________
Bispecific T-cell antibodies and chimeric antigen receptor (CAR) T-cells are highly effective novel therapies in multiple myeloma. These immunotherapies enhance the ability of patient’s own T-cells (a type of immune cell) to recognize and kill myeloma cells. However, some patients do not respond to these treatments, and others relapse after initial response. In some instances, MM cells can mutate and become invisible to T-cells. In addition, T-cells can sometimes become exhausted over time, thereby failing to effectively kill MM cells.
To date, three bispecific antibodies have been FDA-approved for use in multiple myeloma. Two bispecific antibodies, Tecvayli (teclistamab) and Elrexfio (elranatamab), bind to BCMA, a protein target on the surface of MM cells; the third bispecific antibody, Talvey (talquetamab), binds to GPRC5D, a different protein target on the surface of MM cells. All three bispecific antibodies also bind to the CD3 protein on the surface of T-cells, thereby bringing the T-cells into close proximity to the MM cells to initiate MM cell killing by releasing substances (like perforin and granzymes) that destroy MM cells (see Figure 1). These bispecific antibodies are available to patients who have received 4 or more lines of therapy, including proteasome inhibitors (PIs) like Velcade (bortezomib) or Kyprolis, immunomodulatory drugs (IMiDs) such as Revlimid (lenalidomide) or Pomalyst, and anti-CD38 monoclonal antibodies such as Darzalex or Sarclisa. All bispecific antibodies are administered by a subcutaneous (under the skin) injection, usually in the abdominal (stomach) region.
Figure 1.
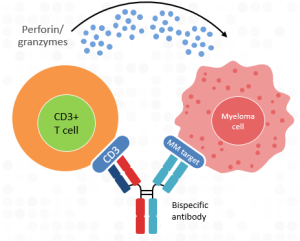
In clinical trials, the two BCMA-targeted bispecifics demonstrated a high overall response rate of ~ 60% in this heavily pre-treated patient population, and progression free survival (the period of time a patient remains in remission after beginning a particular therapy before they relapse) of an average of 11 months. However, patients in high-risk subgroups with high disease burden, such as those whose myeloma cells were growing outside of their bones (also called extramedullary disease or EMD) were less likely to benefit from these therapies.
The most common side effects seen with the anti-BCMA bispecifics include low white blood cell counts (cytopenia), infections, and cytokine release syndrome (CRS), which is a flu-like event that occurs when a patient is first dosed with these agents. CRS severity can be decreased through the use of step-up dosing (meaning the treatment is started with several injections at a lower dose, and the patient is observed in the hospital for 48 hours after each dose for side effects, before the full dose is administered) and by giving low-dose steroids when treatment begins. In addition, dosing these bispecifics less frequently, ie. every 2 weeks instead of weekly, also helps with the severity of these side effects. Interestingly, there are several other BCMA-targeted bispecifics in development which are being tested in clinical trials and may be approved soon.
Talvey, the bispecific antibody that targets GPRC5D on MM cells, has shown an overall response rate of 73% with biweekly dosing, with a lower (49%) response rate in patients with EMD. Side effects from this agent pretty much mirrored what is seen with the BCMA-targeted bispecifics, except that risk of infection is lower with Talvey. Similar to the BCMA-targeted bispecifics, Talvey treatment is administered via a subcutaneous injection in a step-up dosing regimen. There were also additional side effects seen including loss of taste, changes in the fingernails and toenails, and skin rashes/peeling skin. These side effects occur because the cells which are responsible for taste and for nail and skin formation also have GPRC5D on their surface.
Despite how well these drugs work against MM, nearly 1/3 of patients do not respond to them, and those who do respond will eventually relapse, or stop responding. Relapse can sometimes occur due to a high amount of soluble BCMA (sBCMA) floating in the bloodstream of patients who have high disease burden; the anti-BCMA bispecifics can bind to this floating BCMA molecule instead of BCMA on the surface of MM cells, thereby preventing the bispecific from bringing a T-cell into close proximity with a MM cell for killing. One way of decreasing the amount of sBCMA in patients is to block the activity of a molecule called gamma-secretase, which is responsible for releasing BCMA from the surface of MM cells. Researchers are working to discover whether giving patients therapies to decrease their tumor burden before use of a bispecific, or using a bispecific in combination with other backbone therapies such as IMiDs, CELMoDs (a newer class of IMiDs), PIs, or anti-CD38 antibodies, might work to increase how long patients will respond to a bispecific therapy.
The fitness, or killing ability, of T-cells is a critical determinant in how well patients will respond to these therapies. If a patient’s immune system is depleted before beginning this therapy, their T cells may be unable to kill the MM cells even if they are brought into contact with them via the bispecific antibody. While this is primarily the reason why some patients do not respond (are refractory) to bispecific antibodies, it is not usually the reason why patients will relapse from bispecific antibody therapy after responding for some time. Because bispecific antibodies bind to the surface of all T-cells, they can also bind to the surface of another type of T-cell which controls and regulates the immune system; this regulatory T-cell may prevent other T-cells from killing the myeloma cells.
Because MM cells can only be killed by T-cells when a bispecific antibody binds to a protein on their surface, MM cells have been able to escape killing by causing these surface protein “markers” to disappear from their surface, a process known as “antigen escape”. This is primarily why patients relapse from bispecific antibody therapy. Researchers are designing new bispecific antibodies that can bind to MM cells in different ways to keep patients from relapsing. In addition, new studies of patients treated with both Tecvayli and Talvey have shown higher overall response rates (96%), with an 85% response rate in patients with EMD. In addition, there are bispecific antibodies under development that target different MM cell markers such as FcRH5, which may be helpful in treating patients who no longer respond to the other two classes of bispecifics.
In general, data has shown that patients who receive CAR T-cell therapy first and then receive bispecific antibody therapy once they relapse do better than those who receive bispecific antibody therapy first. And, while current strategies have bispecific antibody therapy given until relapse, recent data with cevostamab, a bispecific antibody that targets the FcRH5 protein on the surface of myeloma cells, has shown that fixed duration of therapy followed by stopping treatment can also work for patients who achieve a deep response. For more information on this and other data, see this blog and view this webinar.
In conclusion, bispecifics are an exciting and rapidly evolving new drug class for relapsed/refractory myeloma patients. Further research will help define dosing schedules, duration of therapy, and drug combinations that may work to improve their effectiveness while decreasing their side effects.