One of our MMRF Research Fellows, Dr. Holly Lee of the Arnie Charbonneau Cancer Institute, University of Calgary, Canada, has published an insightful review article about bispecific antibodies in multiple myeloma. We asked her to share her background and help us describe the content of her article.
I am a clinician-scientist in-training. I completed my clinical hematology fellowship in 2021 and have since been enrolled in the Clinician Investigator Program at University of Calgary pursuing a PhD under the supervision of Dr Nizar Bahlis. T-cell redirecting immunotherapies are rapidly transforming how we treat patients with multiple myeloma (MM) in the clinic. However, some patients do not respond to these immunotherapies and others relapse after initial response. My research interests are in understanding the biological mechanisms underlying MM resistance to these novel agents and in developing improved therapeutics that can overcome these barriers.
Receiving MMRF research grant support is invaluable for my career development as an early-career investigator. The funding will help expand our project on investigating tumor intrinsic and extrinsic pathways that drive MM resistance to bispecific T-cell engagers and chimeric antigen receptor T-cell therapies. The insights gained from this research have the potential to optimize immunotherapy selection and sequencing in clinical settings and guide the development of next generation T-cell immunotherapies – areas that will be the primary focus of my research career.
_____________________________________________________________________________________
Bispecific T-cell antibodies and chimeric antigen receptor (CAR) T-cells are highly effective novel therapies in multiple myeloma. These immunotherapies enhance the ability of patient’s own T-cells (a type of immune cell) to recognize and kill myeloma cells. However, some patients do not respond to these treatments, and others relapse after initial response. In some instances, MM cells can mutate and become invisible to T-cells. In addition, T-cells can sometimes become exhausted over time, thereby failing to effectively kill MM cells.
To date, three bispecific antibodies have been FDA-approved for use in multiple myeloma. Two bispecific antibodies, Tecvayli (teclistamab) and Elrexfio (elranatamab), bind to BCMA, a protein target on the surface of MM cells; the third bispecific antibody, Talvey (talquetamab), binds to GPRC5D, a different protein target on the surface of MM cells. All three bispecific antibodies also bind to the CD3 protein on the surface of T-cells, thereby bringing the T-cells into close proximity to the MM cells to initiate MM cell killing by releasing substances (like perforin and granzymes) that destroy MM cells (see Figure 1). These bispecific antibodies are available to patients who have received 4 or more lines of therapy, including proteasome inhibitors (PIs) like Velcade (bortezomib) or Kyprolis, immunomodulatory drugs (IMiDs) such as Revlimid (lenalidomide) or Pomalyst, and anti-CD38 monoclonal antibodies such as Darzalex or Sarclisa. All bispecific antibodies are administered by a subcutaneous (under the skin) injection, usually in the abdominal (stomach) region.
Figure 1.
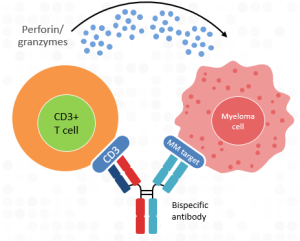
In clinical trials, the two BCMA-targeted bispecifics demonstrated a high overall response rate of ~ 60% in this heavily pre-treated patient population, and progression free survival (the period of time a patient remains in remission after beginning a particular therapy before they relapse) of an average of 11 months. However, patients in high-risk subgroups with high disease burden, such as those whose myeloma cells were growing outside of their bones (also called extramedullary disease or EMD) were less likely to benefit from these therapies.
The most common side effects seen with the anti-BCMA bispecifics include low white blood cell counts (cytopenia), infections, and cytokine release syndrome (CRS), which is a flu-like event that occurs when a patient is first dosed with these agents. CRS severity can be decreased through the use of step-up dosing (meaning the treatment is started with several injections at a lower dose, and the patient is observed in the hospital for 48 hours after each dose for side effects, before the full dose is administered) and by giving low-dose steroids when treatment begins. In addition, dosing these bispecifics less frequently, ie. every 2 weeks instead of weekly, also helps with the severity of these side effects. Interestingly, there are several other BCMA-targeted bispecifics in development which are being tested in clinical trials and may be approved soon.
Talvey, the bispecific antibody that targets GPRC5D on MM cells, has shown an overall response rate of 73% with biweekly dosing, with a lower (49%) response rate in patients with EMD. Side effects from this agent pretty much mirrored what is seen with the BCMA-targeted bispecifics, except that risk of infection is lower with Talvey. Similar to the BCMA-targeted bispecifics, Talvey treatment is administered via a subcutaneous injection in a step-up dosing regimen. There were also additional side effects seen including loss of taste, changes in the fingernails and toenails, and skin rashes/peeling skin. These side effects occur because the cells which are responsible for taste and for nail and skin formation also have GPRC5D on their surface.
Despite how well these drugs work against MM, nearly 1/3 of patients do not respond to them, and those who do respond will eventually relapse, or stop responding. Relapse can sometimes occur due to a high amount of soluble BCMA (sBCMA) floating in the bloodstream of patients who have high disease burden; the anti-BCMA bispecifics can bind to this floating BCMA molecule instead of BCMA on the surface of MM cells, thereby preventing the bispecific from bringing a T-cell into close proximity with a MM cell for killing. One way of decreasing the amount of sBCMA in patients is to block the activity of a molecule called gamma-secretase, which is responsible for releasing BCMA from the surface of MM cells. Researchers are working to discover whether giving patients therapies to decrease their tumor burden before use of a bispecific, or using a bispecific in combination with other backbone therapies such as IMiDs, CELMoDs (a newer class of IMiDs), PIs, or anti-CD38 antibodies, might work to increase how long patients will respond to a bispecific therapy.
The fitness, or killing ability, of T-cells is a critical determinant in how well patients will respond to these therapies. If a patient’s immune system is depleted before beginning this therapy, their T cells may be unable to kill the MM cells even if they are brought into contact with them via the bispecific antibody. While this is primarily the reason why some patients do not respond (are refractory) to bispecific antibodies, it is not usually the reason why patients will relapse from bispecific antibody therapy after responding for some time. Because bispecific antibodies bind to the surface of all T-cells, they can also bind to the surface of another type of T-cell which controls and regulates the immune system; this regulatory T-cell may prevent other T-cells from killing the myeloma cells.
Because MM cells can only be killed by T-cells when a bispecific antibody binds to a protein on their surface, MM cells have been able to escape killing by causing these surface protein “markers” to disappear from their surface, a process known as “antigen escape”. This is primarily why patients relapse from bispecific antibody therapy. Researchers are designing new bispecific antibodies that can bind to MM cells in different ways to keep patients from relapsing. In addition, new studies of patients treated with both Tecvayli and Talvey have shown higher overall response rates (96%), with an 85% response rate in patients with EMD. In addition, there are bispecific antibodies under development that target different MM cell markers such as FcRH5, which may be helpful in treating patients who no longer respond to the other two classes of bispecifics.
In general, data has shown that patients who receive CAR T-cell therapy first and then receive bispecific antibody therapy once they relapse do better than those who receive bispecific antibody therapy first. And, while current strategies have bispecific antibody therapy given until relapse, recent data with cevostamab, a bispecific antibody that targets the FcRH5 protein on the surface of myeloma cells, has shown that fixed duration of therapy followed by stopping treatment can also work for patients who achieve a deep response. For more information on this and other data, see this blog and view this webinar.
In conclusion, bispecifics are an exciting and rapidly evolving new drug class for relapsed/refractory myeloma patients. Further research will help define dosing schedules, duration of therapy, and drug combinations that may work to improve their effectiveness while decreasing their side effects.
Several oral presentations at this year’s American Society of Hematology (ASH) Annual Meeting & Exposition highlighted encouraging outcomes in the treatment of smoldering multiple myeloma (SMM), newly diagnosed MM (NDMM), and relapsed/refractory MM (RRMM). Presentations included updated data on bispecific antibodies (bsAbs) and chimeric antigen receptor T (CAR T)–cell therapy, as well as several novel agents in development.
Recent advancements in the treatment of RRMM have been highlighted in updates from two large CAR T-cell therapy trials: KarMMa-3 and CARTITUDE-2. The KarMMa-3 trial demonstrated the efficacy of idecabtagene vicleucel (ide-cel) in RRMM patients who received 2–4 prior regimens. With 386 patients randomized to receive either ide-cel or standard regimens, ide-cel demonstrated a median progression-free survival (PFS) of 13.8 months and a 51% reduction in the risk of disease progression or death, compared to 4.4 months in the control group. At 18 months, PFS rates were 41% for the ide-cel group versus 19% for standard treatments. The complete response (CR) rate was 44% in the ide-cel arm, with 22% of patients achieving both ≥CR and minimal residual disease (MRD) negativity. The findings indicate an improved response, as well as a longer median time to the next treatment and second PFS, suggesting sustained remission. There were no reported cases of Parkinsonism or Guillain–Barré syndrome.
Additional results from KarMMa-3 reported health-related quality of life (HRQoL) data. Patients receiving ide-cel demonstrated significant benefits in 18 out of 21 HRQoL domains and experienced quicker times to confirmed improvements and prolonged times to deterioration of symptoms, indicating not just clinical but also functional and well-being advantages.
Long-term data on ciltacabtagene autoleucel (cilta-cel) from the CARTITUDE-2 trial, with nearly 29 months of follow-up, showed that all MRD-evaluable patients in cohort A (1–3 prior lines of therapy and lenalidomide-refractory MM) achieved MRD negativity, with 40% maintaining MRD negativity for over 6 months. The overall response rate (ORR) was 95%, with a 24-month PFS and overall survival (OS) rates of 75%. Cohort B, which included patients with early relapse after first-line treatment, showed that 93% reached MRD negativity and an OS rate of 84%. These results suggest that cilta-cel can induce deep and durable responses even in high-risk groups with early relapse.
Findings regarding a wearable device for early cytokine release syndrome (CRS) detection in patients being treated with CAR T-cell therapy were presented. This study showed that the wearable device was superior for monitoring CRS compared with traditional methods. The device, which tracks vital signs such as temperature and heart rate, detected CRS events about 103 minutes earlier than standard care. The study also personalized CRS detection by adjusting temperature thresholds according to each patient’s baseline, leading to even earlier CRS identification. Of note, the device could also detect subclinical CRS events.
A study evaluated the incidence and characteristics of infections in patients treated with B-cell maturation antigen (BCMA)–targeted (teclistamab or elranatamab) and G protein–coupled receptor, class C, group 5, member D (GPRC5D)-targeted (talquetamab) bsAbs. The study included 229 patients across 13 tertiary centers and showed that infections requiring treatment, hospitalization, or treatment delays occurred in a significant portion of the cohort—62%. Notably, infections were more prevalent and severe among those receiving anti-BCMA therapies, with all grade ≥4 events occurring in this subgroup. Infections predominantly affected the pulmonary tract and presented as disseminated infections. Among these, bacterial infections were most common, followed by viral and fungal pathogens. Complications such as invasive pulmonary aspergillosis and progressive multifocal leukoencephalopathy were also reported. The hospitalization rate was 57%, and nearly half of the infectious events affected treatment administration. Of note, the study found that patients treated with anti-GPRC5D bsAbs had a lower risk of first infection compared to the anti-BCMA group, suggesting differential immunosuppressive profiles between these therapies. According to the researchers, dosing interval strategies and prophylaxis appear “necessary and effective” for improving morbidity and mortality rates with these agents.
An analysis from the MonumenTAL-1 trial suggested a benefit for reduced or less-frequent dosing of talquetamab in RRMM patients who achieved partial response (PR) or better, which may help mitigate treatment-emergent adverse events (TEAEs). Results from an analysis of 45 patients who switched to lower-intensity dosing showed that most patients achieved a deepened or maintained response after dose adjustment, with 88.9% sustaining their response 6 months post-switch. Importantly, GPRC5D-associated TEAEs, including oral, nail, and skin-related issues, improved or resolved over time for some patients in the adjusted-dosing cohorts. The authors suggest that “further analyses on the impact of reduced or less frequent talquetamab dosing on clinical outcomes are warranted.”
Another talquetamab study, the phase 1b MonumenTAL-2 study, indicates encouraging results with this agent in combination with pomalidomide. The study included 35 RRMM patients over a median follow-up of 7–11 months. Results indicated a high ORR in both weekly and biweekly dosing cohorts, with deep responses including CRs and very good partial response. In addition, the most common AEs were dysgeusia, CRS, and neutropenia, none of which led to significant treatment discontinuations.
Evaluating prognostic utility of MRD in RRMM patients treated with either CAR T-cell therapy or TCEs, researchers found that sustained MRD negativity was associated with significantly improved survival outcomes. The study involved 269 RRMM patients, 125 treated with CAR T-cell therapy and 144 with TCEs. Out of 509 MRD assessments, patients achieving MRD negativity had a median PFS of 20 months compared to just 3 months in MRD-positive patients. OS was also substantially improved in MRD-negative patients. Notably, MRD negativity rates were higher in patients treated with CAR T-cell therapy compared to TCEs, though the impact of MRD negativity was similar for both CAR T and TCE. In addition, MRD status proved to be an independent prognostic factor for PFS and OS, irrespective of other clinical features, highlighting the importance of MRD negativity as a treatment end point.
Results from a phase 1/2 study highlighted the efficacy of venetoclax (Ven) in combination with daratumumab (D) and dexamethasone (d) in treating patients with t(11;14)-positive RRMM. Initial data showed a 96% ORR, with 67% of patients achieving CR or better. In addition, the combination therapy demonstrated a MRD negativity of 38%, compared to 8% with DVd. Notably, MRD negativity was more durable in the venetoclax group, with some patients maintaining this status for over 12 months.
The combination of sonrotoclax, a BCL2 inhibitor, and dexamethasone showed promising safety and efficacy in RRMM patients with t(11;14). The most common AEs with this combination were insomnia, fatigue, nausea, and arthralgia, none of which were severe. The ORR was 58%, including CRs and VGPRs. At time of data cutoff, 9 patients remained on treatment with the longest duration of response being 483 days (20 cycles). A dose of 640 mg daily in combination with dexamethasone has been recommended for the phase 2 dose.
The phase 2 FUMANBA-1 study evaluating equecabtagene autoleucel (eque-cel, CT103A) in adult RRMM patients after ≥3 prior lines of therapy showed that sustained MRD negativity correlated with improved PFS. Out of 88 patients who achieved MRD negativity, 74 had a follow-up of at least 6 months without progression, and 43 had a follow-up of at least 12 months. Sustained MRD negativity was observed in 78.4% of the patients at 6 months and 74.4% at 12 months. Patients with sustained MRD negativity demonstrated longer PFS than those who did not sustain MRD negativity beyond 6 months. Patients with lower baseline tumor burden and less prior triple-class exposure were more likely to achieve sustained MRD negativity. High-risk cytogenetics, extramedullary disease, performance status, and the number of prior lines of therapy did not differ significantly across MRD subgroups and were comparable to the overall study population.
A phase 1 study evaluated BMS-986393 (CC-95266), a GPRC5D-targeted CAR T-cell therapy, in 70 patients who had received ≥3 prior treatment regimens, including BCMA-directed and CAR T-cell therapies. At a median follow-up of 5.9 months, the ORR was 86% with a CR rate of 38%. In patients refractory to prior BCMA-directed therapies, the ORR was 85% and the CR rate was 46%. TEAEs were mostly hematologic in nature, with 84% of patients experiencing CRS, which was mostly low-grade. Neurotoxicity was observed, with some dose-related events, but were typically reversible. Further research is under way to define the recommended phase 2 dose.
GC012F, a dual-targeting BCMA and CD19 CAR T-cell therapy, has shown efficacy and tolerability in transplant-eligible patients with high-risk NDMM. The phase 1 study, which used the FasTCAR-T platform implementing next-day manufacturing of CAR-T cells, included 22 evaluable patients with high-risk features. The results demonstrated a 100% ORR with a stringent CR rate of 95.5%. Of note, all patients achieved MRD negativity at a sensitivity of 10-6, with sustained MRD negativity at 6 and 12 months. The median duration of response (DOR) and PFS were not reached. Of the patients, 27% experienced low-grade CRS, with no reports of high-grade CRS and no reports of immune effector cell-associated neurotoxicity syndrome (ICANS) or treatment-related deaths.
One-year follow-up from a phase 1 study shows encouraging results with CART-ddBCMA, an autologous CAR-T therapy targeting BCMA with a unique synthetic binding domain. RRMM patients who had received ≥3 prior therapies were treated with a single infusion of CART-ddBCMA following lymphodepletion. As of June 2023, 40 patients with a median age of 66 years were enrolled, and 38 received CART-ddBCMA. The study reported a 100% ORR, with 76% achieving CR or better. Of note, 86% of evaluable patients reached MRD negativity. Of the patients, 95% had CRS, nearly all of which were grade 2 or lower (1 had grade 3 CRS); ICANS was reported in 18% of patients, mostly grade 2 or lower. Median DOR, PFS, and OS were not reached, and the estimated 18-month PFS rate was 67%.
Use of a fractionated initial therapy and booster dose of ARI0002h, a CAR-T cell therapy consisting of a humanized single-chain variable fragment directed at BCMA, showed activity and safety in 60 RRMM patients. Among 60 patients receiving ARI0002h, the ORR in the first 3 months was 95%, with 77% achieving ≥VGPR. MRD-negativity rates on evaluable samples were 98% on day 28 and 96% on day 100. At a median follow-up of 23.1 months, the estimated median PFS was 15.8 months, with no differences based on high-risk cytogenetics or triple-refractoriness. CRS was observed in 90% of patients, with 5% experiencing grades ≥3. ICANS was mild and was reported in 2 patients. A total of 44 out of 55 eligible patients received a booster dose, resulting in 19 patients maintaining stringent CR and 11 improving their response. Median CAR T-cell persistence in peripheral blood was 5 months, with 65% and 52% having measurable CAR T cells at 3 and 6 months, respectively. The authors note that the “booster dose may be partially responsible for the improvement of responses over time and exhaustion may play a role in relapse.”
Results from the phase 1/2 CC-92480-MM-002 trial indicate potential efficacy for mezigdomide (MEZI), a novel oral cereblon E3 ligase modulator. The study evaluated MEZI in combination with dexamethasone (d) and either daratumumab (D) or elotuzumab (E) in patients who had undergone 2–4 previous lines of therapy. In the MeziDd cohort, the ORR was 75%, with a significant portion achieving stringent CRs, CRs, and VGPRs. Similarly, the MeziEd cohort demonstrated good tolerability and promising response rates, especially in patients who had previously received anti-CD38 monoclonal antibody therapy. According to the researchers, these “results support further evaluation of MEZI plus antimyeloma mAbs in phase 1/2 and phase 3 studies.”
HPN217, a BCMA-targeting trispecific T-cell engager, was evaluated in a phase 1 dose-escalation study. HPN217 is a small globular protein, designed to “increase the therapeutic window by minimizing off-target toxicities and CRS.” The study included 97 patients, with 94 treated across various dose levels. Participants had received a median of 6 prior lines of treatment, with a significant proportion being exposed to ≥5 drugs and having undergone transplantation. Most TEAEs were manageable, and the maximum tolerated dose was not reached. CRS was predominantly grade 1–2, and responses were observed at doses ≥2.15 mg, with 55% of efficacy-evaluable patients achieving a PR or better. Among patients with a response, 73% (16/22) have had a confirmed response of VGPR or better. The half-life of HPN217 was median 68 hours, indicating sustained presence in the body. The treatment showed linear and dose-proportional pharmacokinetics, with transient cytokine increases higher with the initial dose compared to subsequent dose.
—
Jointly provided by the MMRF and RedMedEd.
This educational activity is supported by educational grants from AbbVie Inc., Bristol Myers Squibb, and GSK and sponsorship from Genzyme Corporation and Legend Biotech USA Inc.
Several oral presentations at this year’s American Society of Hematology (ASH) Annual Meeting & Exposition highlighted encouraging outcomes in the treatment of smoldering multiple myeloma (SMM), newly diagnosed MM (NDMM), and relapsed/refractory MM (RRMM). Presentations included updated data on bispecific antibodies (bsAbs) and chimeric antigen receptor T (CAR T)–cell therapy, as well as several novel agents in development.
Adding daratumumab to lenalidomide, bortezomib, and dexamethasone (RVd) for patients with NDMM appears to improve treatment outcomes, according to the finding of a real-world comparison study. The study compared daratumumab-RVd (D-RVd) and RVd induction therapy, evaluating response rates and long-term survival for both standard- and high-risk patients. The cohort study included over a thousand patients treated with RVd and 326 patients treated with D-RVd induction therapy. Patients undergoing D-RVd showed an overall response rate (ORR) of 99.6% post-induction and an 86.5% rate of very good partial responses or better (≥VGPR). These rates were higher than the RVd cohort (97.1% ORR; 67.6% ≥VGPR). Although the median follow-up for the D-RVd cohort was shorter, a clear progression-free survival (PFS) advantage was observed for both standard- and high-risk groups. At the 2-year mark, PFS for D-RVd patients was 93%; it was 82% for those on RVd. Moreover, overall survival (OS) rates also favored the D-RVd group, particularly in high-risk patients, with 94% in D-RVd versus 79% in the RVd cohort. According to the researchers, “D-RVd is a highly effective induction regimen that can improve upon outcomes in a historical NDMM population treated with RVd in terms of depth of response and PFS benefit.”
The late-breaking abstracts session included results from the phase 3 PERSEUS trial, another study evaluating the D-RVd regimen for NDMM patients who were eligible for autologous stem cell transplantation (ASCT). The primary results demonstrated that adding subcutaneous daratumumab to RVd significantly improved patient outcomes. The trial included 709 patients aged 18–70 who were randomized to receive D-RVd followed by maintenance therapy with either D-R or R alone. At a median follow-up of 47.5 months, the D-RVd arm demonstrated an improved PFS compared to RVd alone, with an estimated 48-month PFS rate of 84.3% versus 67.7%. ORR (≥CR) and minimal residual disease (MRD) negativity were significantly higher (P<0.0001 for both) in the D-VRd group. Results were consistent across all subgroups, including in patients with high cytogenetic risk and International Staging System (ISS) stage III disease. According to the researchers, the safety profile remained consistent with known profiles for RVd, with no new safety concerns reported. From these results—in addition to data from the previous phase 2 GRIFFIN trial—the researchers concluded, “these data…demonstrate the consistent and clinically meaningful benefit of quadruplet (daratumumab) plus RVd followed by D-R maintenance versus triplet RVd followed by lenalidomide maintenance and support [this combination] as a new standard of care for transplant-eligible NDMM.”
The phase 3 IsKia study evaluated isatuximab, carfilzomib, lenalidomide, and dexamethasone (IsaKRd) versus KRd in 302 transplant-eligible NDMM patients. The MRD negativity rate (10-5) after consolidation (the study’s primary end point) was 77% with IsaKRd versus 67% with KRd (P=0.049). The benefit of MRD negativity from IsaKRd treatment versus KRd was consistent across all patient subgroups. MRD negativity rates and subgroup analysis favored IsaKRd following induction and after ASCT, as well. At a median follow-up of 20 months, PFS was no different between treatment arms. More patients experienced grade ≥1 hematologic and non-hematologic toxicities with IsaKRd (55% and 41%) than KRd (43% and 37%).
Results from the first interim analysis of the phase 2 EMN26 study indicate that iberdomide, a novel oral cereblon E3 ligase modulator, shows promise as a maintenance therapy following ASCT in NDMM patients. The multicohort study explored the safety and clinical activity of iberdomide in patients who had achieved a partial response or better post-ASCT. A total of 111 patients in 3 dosage cohorts (0.75, 1.0, or 1.3 mg) were enrolled, with 69 patients receiving ≥6 cycles of treatment (n=34 in 1.0-mg cohort; n=35 in 1.3-mg cohort; n=0 in 0.75-mg cohort [this cohort was added later]). The data showed a significant deepening of response across the cohorts, with a reported 48% response improvement in the 1.0-mg cohort and 45% in the 1.3-mg cohort—which was significantly higher than the null hypothesis of ≤20% improvement within 6 months. The most common grade ≥3 adverse events were neutropenia, infections, and fatigue/asthenia. According to the researchers, “iberdomide represents a novel effective post-ASCT maintenance strategy with a favorable safety profile and superior response improvement at 6 months than what has been observed with lenalidomide maintenance (26% at 6 months in the EMN02 study).”
Results from the OPTIMUM/MUKnine trial suggest that risk of early relapse is increased in patients with ultra-high-risk (UHiR) MM with multi-hit tumors and a SKY92 high-risk signature, even with aggressive treatment. The study included UHiR MM patients characterized by ≥2 high-risk cytogenetic abnormalities (HRCAs) and/or the SKY92 high-risk signature or primary plasma cell leukemia. Patients underwent intensive therapy, including with Dara-CVRd, V-ASCT, and extended Dara-VR(d) consolidation. Despite this, a subset of participants relapsed within the first 18 months. In an exploratory analysis, the baseline characteristics of patients who experienced early relapse were compared against those who did not. Lower platelet levels were more common in the early relapse group, and a majority were at ISS stage 2. By contrast, only one out of 29 ISS stage 1 patients faced early relapse.
The presence of del(17p) was also significantly associated with early relapse, with 50% of these patients relapsing early. Of note, most patients with del(17p) myeloma relapsing early also had a SKY92 high-risk signature, whereas the majority of patients with del(17p) myeloma who did not relapse early were SKY92 standard risk. Regarding MRD status at the end of induction, the presence of soft tissue plasmacytomas was the only clinical factor associated with an insufficient response; ISS stage was not. Most patients identified as SKY92 high risk alone remained MRD-positive. The results highlight the “utility of integrated molecular diagnostics, including gene-expression risk profiling, to support clinical decision-making in the era of advanced frontline combination therapies.”
Also in high-risk patients, diffusion-weighted magnetic resonance imaging (DW-MRI) outperformed positron emission tomography (PET-CT) in detecting residual focal lesions post-ASCT. Most NDMM patients achieve deep responses during first-line therapy but end up relapsing due to focal lesions acting as reservoirs for relapsed disease. However, the best imaging modality for tracking focal lesions remains unclear. PET-CT and DW-MRI were evaluated at multiple predefined time points to track resistant lesions in 170 NDMM patients. The findings indicated that DW-MRI outperformed PET-CT. Though both imaging modalities initially identified focal lesions in most patients, DW-MRI showed a substantially higher detection rate post-ASCT, finding lesions in 53 patients compared with only 12 detected by PET-CT. Patients without focal lesions at baseline as determined by both PET-CT and DW-MRI remained negative post-ASCT. In addition, with the exception of 1 patient, focal lesions after ASCT were persistent from initial diagnosis. About two thirds of persistent post-transplant lesions were located in the humeri or femora—which was unexpected given that myeloma is considered a disease of the axial skeleton, according to the researchers.
HSCT is a standard of care for eligible MM patients and has been shown to improve survival. A comprehensive examination of the National Cancer Database from 2004 to 2020 of 43,653 patients recommended for HSCT found a refusal rate of approximately 2%. Those who underwent HSCT after induction chemotherapy had a median OS of 124 months compared to 95 months for those who declined. The research identified several factors influencing HSCT refusal:
According to the researchers, HSCT refusal rates have increased over the years, potentially due to the emergence of novel therapies and anti-CD38 immunotherapy, providing alternative treatment options.
An international study from the Worldwide Network for Blood & Marrow Transplantation was conducted between 2013 and 2017 and included 61,725 patients to estimate how age influences post-transplant survival and relapse rates. The study found that <1% of patients >75 receive an ASCT, pointing to a potential age bias in treatment selection. In addition, the use of conditioning with melphalan 200 mg/m2 was predominant among younger patients, with a shift to a lower dose of 140 mg/m2 in patients >75. As expected, OS and PFS were lower in older age groups. However, the incidence of relapse did not significantly vary with age, suggesting that disease course post-transplant is relatively consistent across age groups. Non-relapse mortality was significantly higher with advancing age, though it remained very low even in patients >75.
—
Jointly provided by the MMRF and RedMedEd.
This educational activity is supported by educational grants from AbbVie Inc., Bristol Myers Squibb, and GSK and sponsorship from Genzyme Corporation and Legend Biotech USA Inc.
Several oral presentations at this year’s American Society of Hematology (ASH) Annual Meeting & Exposition highlighted encouraging outcomes in the treatment of smoldering multiple myeloma (SMM), newly diagnosed MM (NDMM), and relapsed/refractory MM (RRMM). Presentations included updated data on bispecific antibodies (bsAbs) and chimeric antigen receptor T (CAR T)–cell therapy, as well as several novel agents in development.
The use of the bsAb teclistamab in patients with high-risk SMM was evaluated as part of the Immuno-PRISM trial, which examined whether bsAb therapy provided greater benefit in SMM than RRMM patients due to SMM patients having a more intact immune system and lower disease burden. Patients (n=12) received teclistamab alone or lenalidomide and dexamethasone. Teclistamab was associated with grade ≥3 hematologic toxicities, including neutropenia in 4 patients and thrombocytopenia in 1. Grade ≥3 non-hematologic toxicities included an increase in alanine transaminase (ALT) in 3 patients and pancreatitis in 1. Infections of any grade occurred in 9 patients, with one grade 3 case of sinusitis in 1 patient. Cytokine release syndrome (CRS) occurred in 75% of patients, mostly grade 1. No patients experienced immune effector cell–associated neurotoxicity syndrome (ICANS) or delayed neurotoxicity. Overall response rate (ORR) was 100%, with 42% complete response (CR), 25% very good partial response (VGPR), and 33% partial response (PR).
Four patients with high-risk FISH receiving teclistamab achieved a CR within 5 cycles. These data were superior to those of a control arm of lenalidomide and dexamethasone (n=3), in which the ORR was 66% with no CRs so far. In patients treated with teclistamab, the minimal residual disease (MRD) negative rate at 10-6 was 100% with no progression on treatment.
The final analysis of the phase 2 CENTAURUS study demonstrated long-term efficacy and safety for daratumumab monotherapy in patients with intermediate- or high-risk SMM. Conducted over a follow-up period extending to approximately 7 years, the study highlights the potential for daratumumab to delay the progression to MM in SMM patients who had been diagnosed for <5 years. Patients were randomized to receive daratumumab on one of three dosing schedules: intense (weekly in cycle 1, every 2 weeks in cycles 2 and 3, every 4 weeks in cycles 4–7, and every 8 weeks in cycles 8–20), intermediate (weekly in cycle 1 and every 8 weeks in cycles 2–20), and short (weekly 1 cycle only). A higher ORR and ≥CR rate was observed in the intense and intermediate arms, with a combined ≥CR rate of 8.5%. In addition, the 84-month overall survival (OS) rate was >80% across all dosing arms, with median OS not reached in any arm. Grade 3/4 treatment-emergent adverse events (TEAEs) were most frequent in the intense arm but were generally manageable across all groups. No new safety concerns arose from the extended follow-up.
A phase 2 study conducted by the National Institutes of Health highlights promising long-term results in patients with high-risk SMM (HR-SMM) treated with a combination of carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide maintenance (KRd-R). The study aimed to investigate the durability of responses off therapy, following all patients completing lenalidomide maintenance. Patients with HR-SMM were enrolled based on specific risk criteria and underwent an induction phase with KRd-R followed by a 2-year lenalidomide maintenance phase.
At a median follow-up of >60 months:
The researchers concluded from these findings that “patients who achieve MRD-negative remissions after induction therapy have prolonged biochemical PFS,” although further follow-up is needed to fully understand the rates of PFS and OS.
—
Jointly provided by the MMRF and RedMedEd.
This educational activity is supported by educational grants from AbbVie Inc., Bristol Myers Squibb, and GSK and sponsorship from Genzyme Corporation and Legend Biotech USA Inc.
Day 2 of ASH represented the calm before the storm, as only a couple of abstracts on myeloma were featured on Sunday compared to the more than a dozen presentations that will be highlighted on Day 3. Updates from Day 2 included a real-world comparison of quadruplet versus triplet regimens in standard- and high-risk newly diagnosed multiple myeloma and an assessment of the real-world utilization of autologous stem cell transplantation (ASCT) in newly diagnosed patients.
Preferred treatments for induction therapy (the first in a series of treatments used to treat multiple myeloma) typically consist of three-drug (triplets) or four-drug (quadruplets) regimens given over three to six cycles, each of which typically lasts 3 or 4 weeks. The combination of Revlimid (lenalidomide)-Velcade (bortezomib)-dexamethasone (RVd) is highly effective for patients with NDMM. However, the addition of Darzalex (daratumumab) to RVD (D-RVD) has shown improved depth of response and trend towards a benefit of progression free survival (PFS)—that is, the length of time during and after treatment in which a patient is living with a disease that does not get worse.
In this presentation, Dr. Nisha Joseph and colleagues from Emory University (Abstract 647) analyzed the real-world response rates and long-term outcomes for both standard- and high-risk patients. High-risk disease was defined as having chromosomal alterations including del(17p), t(4;14), and t(4;16). The real-world analysis included 1000 NDMM patients treated with RVD and 326 NDMM patients treated with D-RVD induction therapy. The results showed:
Dr Joseph and colleagues concluded that D-RVD is a highly effective induction regimen that can improve upon outcomes in a historical NDMM population treated with RVD in terms of depth of response and PFS benefit. This analysis provides evidence of benefit with the addition of daratumumab to RVD in increasing depth of response and provides an early glimpse of the promising PFS and OS benefit not only in standard risk patients, but also in patients with high-risk cytogenetic and disease features.
High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) remains standard of care in multiple myeloma (MM) in eligible patients and is proven to improve progression free survival. However, some patients do not receive ASCT as part of their treatment. It is well documented that racial and socioeconomic disparities in the use of ASCT exist in myeloma treatment. Barriers to patients receiving ASCT as part of their treatment may include low income, insurance status, and poor access to care at an academic health center. In this presentation (Abstract 532), Dr. Chakra Chaulagain and colleagues at the Cleveland Clinic Florida evaluated data from the National Cancer Database and found that the ASCT opt out rate in real-world is low (approximately 2%) but still represents a missed opportunity to provide standard of care for myeloma patients. Older patients (aged over 60 years), females, African American patients with non-private health insurance (that is, those covered by Medicaid, Medicare, or another government insurance), higher comorbidities, and those with an income of less than $63,000 per year were found to be less likely to receive an ASCT.
Among 43,653 patients evaluated in the study, those treated at non-academic medical centers were less likely to receive ASCT than patients treated at academic facilities. Patients living in the South Atlantic region of the United States were less likely to receive an ASCT compared to other regions of the country. The researchers conclude that their findings reveal significant racial, economic, and geographic variation regarding the use of ASCT across the US which should be further studied. Understanding the barriers to the use of ASCT is crucial for optimizing patient care and tailoring effective interventions.
Be sure to hear what myeloma experts Dr. Nisha Joseph and Dr. Alexander Lesokhin, had to say about the day’s presentations here.
Stay tuned for more updates from day 3 at ASH 2023!
Welcome to our 2023 recap of the latest findings on myeloma treatments reported at the American Society of Hematology (ASH) meeting that kicked off Saturday in San Diego. Highlights from today included real-world data on the use of the bispecific antibody Tecvayli, health-related quality of life findings with the CAR T-cell therapy Abecma, the use of minimum residual disease negativity, iberdomide maintenance therapy, and updates on the treatment of high-risk newly diagnosed disease and smoldering myeloma.
Tecvayli (Teclistamab) was the first “off-the shelf” BCMA-targeted bispecific antibody approved for patients with heavily pretreated relapsed/refractory multiple myeloma (RRMM; that is patients who have received 4 or more lines of therapy), including a proteasome inhibitor (PI), an immunomodulatory drug (IMiD), and an anti-CD38 monoclonal antibody. In this Abstract 91, Dr. Danai Dima and colleagues from the Cleveland Clinic reported that treatment with Tecvayli in a real-world setting showed similar responses to the previously reported data from the MajesTEC-1 clinical trial without any new side effects.
Of the 102 patients with RRMM included in Dr. Dima’s analysis, 25% were non-Hispanic Black, 44% had extramedullary disease (EMD; that is, myeloma cells that are growing outside of the bone marrow) and more than 80% (83/102) would not have met the MajesTEC-1 eligibility criteria. Their findings showed:
The authors note that results from the real-world study of the safety and efficacy of Tecvayli were like previously reported data from the MajesTEC-1 clinical trials; however, complete response (CR) rates were lower. The lower CR rates may be due to the fact that a majority of patients evaluated did not meet the eligibility criteria MajestTEC-1 trial population; due to stringent eligibility criteria, patients in clinical trials tend to be less sick and/or have fewer comorbidities than real-world patient population, so outcomes are typically better in clinical trials. Low blood cell counts and infections remain a challenge, thus, close monitoring, supportive care and other measures are important to help patients continue with therapy.
Dr. Michel Delforge and colleagues in Belgium reported findings from a preliminary analysis (Abstract 96) that showed the chimeric antigen receptor T cell (CAR T-cell) therapy Abecma (idecabtagene vicleucel) significantly improved health-related quality of life when compared to standard regimens [that is, Darzalex (dara, daratumumab), Pomalyst (pomalidomide), and dexamethasone; Darzalex, Velcade (bortezomib), and dexamethasone; Ninlaro (ixazomib), Revlimid, and dexamethasone; Kyprolis (carfilzomib) and dexamethasone; or Empliciti (elotuzuamb), Pomalyst, and dexamethasone].
Researchers evaluated data from patient-reported outcomes that were collected as part of the KarMMa-3 trial. In the phase 3 trial, Abecma significantly improved progression-free survival (PFS) and treatment response rates as compared with standard regimens, in patients with triple-class exposed (TCE) RRMM who had received 2–4 prior regimens (Rodriguez-Otero P, et al. N Engl J Med 2023).
Health-related quality of life captures information on the physical and mental health status of individuals, and on the impact of disease and treatment on a patient’s quality of life. The results from the patient-reported outcomes showed statistically significant and clinically meaningful improvements in health-related quality of life including cognitive functioning, fatigue, and pain reduction for patients with RRMM who received Abecma compared with standard treatment regimens. The authors noted that health-related quality of life improvements occurred earlier with Abecma therapy than with standard regimens, starting at approximately 2-3 months following infusion and were sustained for more than 2 years.
A couple of abstracts evaluated the role of minimal residual disease (MRD) as a tool to measure the success of therapy. MRD is an important topic in the field of multiple myeloma, in large part because we have very active treatment regimens that can bring deep and sustained responses to patients, something that was not possible just several years ago. Measuring MRD refers to counting the number of multiple myeloma cells that remain in a patient after a course of therapy is completed. For some patients, achieving MRD negativity (that is, no disease was detected after treatment) is associated with a significantly longer time before disease progression (progression-free survival [PFS]) and overall survival; however, to date, there has been limited information about its clinical meaning in patients treated with CAR T-cell therapy.
In the first presentation, (Abstract 94), Dr. Aintzane Zabaleta and colleagues from Spain found that achieving sustained MRD negativity resulted in significantly prolonged survival of RRMM patients treated with newer immunotherapies such as CAR T-cell therapy and bispecific antibodies (also known as T-cell engagers). The researchers reported that MRD negativity was associated with 88% reduction in the risk of progression and/or death. MRD negative rates were significantly higher in patients treated with CAR T-cell therapy (78%) than bispecific antibody therapy (35%).
In the next abstract, Nizar Bahlis, MD and colleagues from Canada reported (Abstract 338) that Venetoclax (Ven) combined with daratumumab (D) and dexamethasone (d) [VenDd] showed higher rates of MRD-negativity and sustained MRD-negativity compared to bortezomib plus Dd (DVd) in patients with t(11;14)-positive RRMM.
Venetoclax is a potent and selective oral BCL-2 inhibitor with demonstrated anti-myeloma activity in patients with t(11;14)-positive RRMM. The combination of VenDd has shown a high overall response rate and tolerable safety profile in the early phase study. The results in 81 patients showed:
The authors concluded that VenDd treatment showed higher rates of MRD-negativity and sustained MRD-negativity compared to DVd in patients with t(11;14)-positive RRMM.
Maintenance therapy with Revlimid (lenalidomide) is the standard of care following induction therapy and ASCT; however, all patients are at risk of relapse following transplantation, and up to 30% stop Revlimid maintenance therapy due to intolerable side effects. Thus, new treatment options with improved activity and tolerability are needed for maintenance therapy.
Iberdomide is a novel, potent oral cereblon E3 ligase modulator (CELMoD™), which is similar to but more potent than Revlimid, with a dual function: activate the immune system and directly kill myeloma cells by inducing the destruction of proteins that drive cancer growth. Researchers from the Netherlands reported (Abstract 208) that iberdomide maintenance therapy following ASCT showed an improvement in response over time in patients who received IMiD/PI-based induction with or without anti-CD38 antibody therapy and ASCT. Researchers noted that:
The most common serious side effects observed with iberdomide were low blood cell counts, infections, and fatigue.
The researchers conclude that iberdomide represents a novel effective post-ASCT maintenance strategy with a favorable safety profile and superior response improvement at 6 months than what has been observed with Revlimid maintenance. Iberdomide is currently being studied (versus Revlimid) as maintenance therapy following ASCT in a phase 3 trial.
While observation is considered standard of care for smoldering multiple myeloma (SMM) patients who have been identified as having a high risk of progressing to active myeloma, therapeutic intervention at the SMM stage may help delay the progression to MM.
Dr. Ola Landgren, and colleagues from the University of Miami presented the final analysis of the phase 2 CENTAURUS study that showed Darzalex monotherapy demonstrated clinical activity in 123 patients with Intermediate-Risk or High-Risk SMM after a median follow-up of approximately 7 years (Abstract 210).
The researchers examined 3 different dosing schedules (that is, long intense; intermediate; short intense) to determine the optimal schedule of treatment administration for the phase 3 AQUILA study. At a median follow up of 85 months, the study found:
The researchers conclude that this final analysis of CENTAURUS continue to demonstrate the clinical activity of DARA monotherapy in patients with intermediate- or high-risk SMM after a median follow-up of about 7 years, which supports the ongoing phase 3 AQUILA study and future SMM trials.
Data presented at the International Myeloma Society (IMS) annual meeting held September 27–30 in Athens, Greece, focused on 3 main topics: (1) the use of newer agents, (2) optimizing outcomes, and (3) the use of minimal residual disease (MRD) measurements to predict long-term outcome for patients or to potentially stop maintenance therapy.
Patients achieving sustained MRD negativity at 3 years post-maintenance initiation may be able to discontinue lenalidomide maintenance, according to data presented at IMS. The study enrolled 151 NDMM patients who underwent ASCT, with 42 patients successfully discontinuing lenalidomide maintenance after 3 years of sustained MRD negativity. The researchers found that MRD negativity was maintained in most patients at various time points post-discontinuation. Six months after discontinuation of lenalidomide maintenance, 39 out of 41 patients were MRD negative. At 12 months, 36 out of 38 patients continued to be MRD negative. At 18 months, all evaluable patients (n=18) remained MRD negative. At 24 months, 13 out of 14 patients were MRD negative, and at 30 months all 4 evaluable patients were MRD negative. Overall, 5 patients restarted treatment with lenalidomide monotherapy after converting from MRD negative to MRD positive following the initial completion of maintenance. One patient progressed and received second-line treatment. “Sustained MRD negativity after 3 years of lenalidomide maintenance may guide the safe discontinuation of maintenance, though this has to be proven in prospective randomized clinical trials,” the researchers note.
A predictive model involving 3 measurable risk factors in MM—ISS stage, circulating tumor cell (CTC) levels, and time to first MRD negativity—may help identify TE patients with risk factors that can predict disease recurrence. A total of 267 patients out of 458 who attained MRD negativity by next-generation flow cytometry were analyzed over a median follow-up of 73 months. The patients in this analysis had been enrolled in the GEM2012MENOS65/GEM2014MAIN clinical trials. Results showed that 54% of patients maintained MRD negativity, 42% experienced MRD resurgence or progressive disease, and 4% died without progression. Prognostic factors at diagnosis, including ISS stage III and ≥0.01% CTCs, were found to predict MRD resurgence or progression, whereas patients achieving MRD negativity sooner, particularly after induction (<6 months), exhibited a lower risk of MRD resurgence or progression. A dynamic model incorporating ISS stage, CTC levels, and time to first MRD negativity assessment demonstrated predictive potential. Five-year rates of MRD resurgence or progression in patients with no, one, or ≥2 risk factors were 16%, 33%, and 57%, respectively. According to the researchers, this model could aid in both clinical trial design and routine practice decision-making.
Serial MRD testing within the first 5 years after diagnosis may help predict long-term outcomes. Data presented at IMS included 1,744 NDMM patients who underwent single or tandem ASCT. Patients were categorized into 3 groups based on their MRD test results in the initial 5 years of treatment. Group 1, which included patients with 3 serial MRD-negative tests, demonstrated an encouraging long-term outcome, with a 10-year PFS of 74%. By contrast, Group 2 included patients with both negative and positive MRD tests and exhibited a 10-year PFS of 30%. Group 3, with consistent MRD-positive tests, had a 10-year PFS of 1%. This study suggests that achieving and maintaining serial MRD negativity in the early years of myeloma treatment may predict excellent long-term outcomes, offering potential guidance for clinical practice and trials. According to the researchers, “achievement of 3 serial MRD-negative tests in the first 5 years of therapy is predictive of an excellent long-term outcome with few treatment failures.”
——————————————————
Jointly provided by the MMRF and RedMedEd.
This educational activity is supported by educational grants from AbbVie Inc., Bristol Myers Squibb, and GSK, and sponsorship from Legend Biotech USA Inc.
Data presented at the International Myeloma Society (IMS) annual meeting held September 27–30 in Athens, Greece, focused on 3 main topics: (1) the use of newer agents, (2) optimizing outcomes, and (3) the use of minimal residual disease (MRD) measurements to predict long-term outcome for patients or to potentially stop maintenance therapy.
In a study evaluating 2 vs 4 years of monthly intravenous ZOL, 192 symptomatic NDMM patients were randomized after 2 years ZOL to either 2 additional years of monthly IV ZOL or observation. The 2 additional years of ZOL were significantly superior in protecting against progressive bone disease (PBD): 8 cases were reported in the ZOL arm and 18 cases in the observational arm (HR: 0.38, 95% CI [0.17-0.88], P=0.024). In addition, there was no statistically significant difference in either osteonecrosis of the jaw (ONJ) incidence or OS between the 2 groups. According to the researchers, 79% of patients had bone involvement at diagnosis and 59% experienced bone pain. ZOL may help prevent PBD but is associated with ONJ, particularly when administered over a longer duration or with greater potency.
The final OS analysis of the OPTIMISMM trial comparing PVd vs Vd alone in patients with lenalidomide-refractory RRMM was reported. This randomized open-label phase 3 trial showed a nonsignificant trend towards improved OS with PVd (35.6 vs 31.6 months, respectively). During the study, 71% vs 70% died in the PVd and Vd groups, respectively. However, PFS was significantly improved with PVd versus Vd (22.1 vs 16.9 months; HR [95% CI], 0.77 [0.64–0.94]; P=.008). Time to treatment failure was also longer with PVd versus Vd (8.8 vs 4.6 months). The most common TEAEs with PVd were neutropenia (54%), peripheral sensory neuropathy (48%), and thrombocytopenia (40%); with Vd, the most common TEAEs were thrombocytopenia (39%), peripheral sensory neuropathy (38%), and diarrhea (31%). Peripheral neuropathy was the most common TEAE that resulted in discontinuation (PVd, 11%; Vd, 8%). According to the researchers, these data support the use of PVd as an effective treatment option in patients with RRMM.
The GMMG-CONCEPT trial evaluated the quadruplet isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in both transplant-eligible (TE) and ineligible (TNE) patients with newly diagnosed high-risk MM (HRMM), defined International Staging System (ISS) stage 2 or 3 and HRCA such as del17p, t(4;14), t(14;16), or >3 copies 1q21 (amp1q21). Results presented at IMS indicated substantial MRD negativity after consolidation, with rates of 67.7% for TE patients and 54.2% for TNE patients. The current analysis includes 127 TE and 26 TNE patients with sustained MRD negativity and PFS. After a median follow-up of 40 months for TE patients and 33 months for TNE patients, the median PFS had not yet been reached in either study arm. Exploratory analyses revealed promising 1-year and 2-year PFS rates for both groups. Additional subgroup analyses showed that patients with elevated lactate dehydrogenase (LDH) or ≥2 HRCAs or del17p were least likely to reach MRD negativity and had shortened PFS. The researchers reported that Isa-KRd induces high rates of sustained MRD negativity in newly diagnosed HRMM, translating to a median PFS that was not yet reached. The results of this study are now published and can be found here.
——————————————————
Jointly provided by the MMRF and RedMedEd.
This educational activity is supported by educational grants from AbbVie Inc., Bristol Myers Squibb, and GSK, and sponsorship from Legend Biotech USA Inc.
Data presented at the International Myeloma Society (IMS) annual meeting held September 27–30 in Athens, Greece, focused on 3 main topics: (1) the use of newer agents, (2) optimizing outcomes, and (3) the use of minimal residual disease (MRD) measurements to predict long-term outcome for patients or to potentially stop maintenance therapy.
CC-220-MM-001 is an ongoing phase 1/2 trial of iberdomide, used alone and in various combinations, in patients with both relapsed/refractory (RRMM) and newly diagnosed (NDMM) multiple myeloma. Iberdomide-dexamethasone in RRMM, as published here, showed clinical activity in heavily pretreated patients, including those with immunomodulatory-refractory disease.
The initial results of iberdomide, bortezomib, and dexamethasone (IberVd) in 18 transplant-ineligible NDMM patients demonstrated an 88.9% (95% CI, 65.3-98.6) overall response rate (ORR) in the intent-to-treat population, with 4 stringent complete responses (sCRs), 5 CRs, 5 very good partial responses (VGPR), and 2 partial responses; 2 patients were not evaluable. Overall, 9 (50.0%) patients achieved at least a CR and 14 (77.8%) patients achieved a VGPR or better. Grade 3/4 treatment-emergent adverse events (TEAEs) were noted in 70.6% of patients. Neutropenia and pneumonia were the most common AEs (17% each). No patients discontinued due to AEs, however. The researchers reported that the combination showed “high efficacy with deep, ongoing responses in this cohort of mostly older patients… supporting further assessment of iberdomide combinations in the frontline setting.” Iberdomide is currently being studied (versus lenalidomide) as maintenance therapy following autologous stem cell transplant (ASCT) in a phase 3 trial.
Mezigdomide was combined with either bortezomib and dexamethasone (MeziVd) or carfilzomib and dexamethasone (MeziKd) in RRMM patients who had received 2 to 4 prior regimens or MeziVd-1.0 mg in RRMM patients who had received 1 to 3 prior regimens in the phase 1/2 CC-92480-MM-002 trial. The most common grade 3/4 TEAEs were hematologic and included neutropenia and thrombocytopenia with MeziVd and neutropenia and infections with MeziKd or MeziVd-1.0 mg. Dose reductions of mezigdomide due to TEAEs were required in 25% to 40% of patients, depending on the dose and combination. The ORR was 75.0% with MeziVd (21/28); 84.2% with MeziVd-1.0 mg (32/38); and 85.2% with MeziKd (23/27). Median time to response (range) was 1.38 (0.7-3.3), 0.89 (0.7-2.4), and 0.95 (0.9-5.1) months in the MeziVd, MeziVd-1.0 mg, and MeziKd cohorts, respectively. Median duration of response was 10.4 and 11.9 months in the MeziVd and MeziKd cohorts and was not reached in the MeziVd-1.0 mg group. These data support further exploration of mezigdomide in phase 3 studies. Mezigdomide is currently being studied in the SUCCESSOR-1 and -2 phase 3 trials.
Venetoclax, a potent oral BCL-2 inhibitor, has the potential to be the first biomarker-directed therapy for patients with RRMM positive for the t(11;14) translocation, who tend to exhibit higher BCL-2 levels.
In an ongoing phase 2 study, venetoclax, carfilzomib, and dexamethasone (VenKd) was used to treat patients with t(11;14)-positive RRMM. Patients were randomized 5:3:5 to receive K (70 mg/m2 weekly) and d (40 mg) in combination with daily venetoclax (400 mg or 800 mg) or Kd alone. The current analysis included 56 patients. The most common TEAEs, occurring in at least half of patients, were diarrhea, nausea, fatigue, and vomiting and were more common in venetoclax-treated patients than in those treated with Kd alone. Grade ≥3 TEAEs occurring in at least 20% in any group were lymphopenia, neutropenia, and hypertension. Grade ≥3 infection rates were higher in the VenKd groups vs Kd alone (29% vs 20% vs 11%). After a median follow-up of approximately 1–2 years, ORRs (95% CI) were 94% (71-100), 95% (75-100), and 58% (34-80) in the venetoclax 400 mg, 800 mg, and Kd-alone groups, respectively. CR/sCR rates were 29, 50, and 11%, respectively. Median time to response was 1.0, 1.0, and 2.4 months, and 12-month progression-free survival (PFS) estimates were 67, 85, and 79%, with median PFS of 42.4 months. Overall survival (OS) data are not yet mature. Study enrollment is ongoing. According to the researchers, treatment with VenKd was well tolerated and produced favorable responses in at least 90% of patients.
The CANOVA study compared once-daily oral venetoclax and dexamethasone (Vd) versus pomalidomide and dexamethasone (Pd) in 263 patients 18 years and older who had t(11;14)-positive RRMM and had received ≥2 prior lines of therapy. Vd did not significantly improve PFS relative to Pd, the primary end point of the trial. Patients receiving Vd showed a median PFS of 9.9 months compared with 5.8 months with Pd (HR = 0.823, 95% CI: [0.596, 1.136]; P=0.237). The safety profile of Vd was generally consistent with the known safety profiles when used as single agents, and no new safety signals emerged. The most common AEs in the Vd group were infection (61%), diarrhea (41%), lymphopenia (24%), and nausea (22%). The most common AEs in the Pd group were neutropenia (63%), infection (57%), thrombocytopenia (39%) and anemia (35%).
Bispecific antibodies (bsAbs) that target B-cell maturation antigen (BCMA) may contribute to increased infection risk in RRMM patients and warrant preventive measures against infection.
To devise recommendations for clinical practice, researchers analyzed data from the phase 1/2 MajesTEC‑1 study of teclistamab, a BCMA×CD3 bsAb, involving 165 RRMM patients. After a median follow-up of 21.7 months, approximately 78% of patients (n=129) developed infections, with over half of the overall study participants developing grade 3/4 infections. Twenty patients (12.1%) died due to infections (17 had COVID-19). Median time to first onset of any grade infection was 1.7 months. Overall, 70.9% of patients had at least 1 IgG value <400 mg/dL and 45.5% received intravenous immunoglobulin (IVIG). Grade 3/4 neutropenia occurred in 65.5% of patients at a median of 2.3 months, and 53.3% of patients received granulocyte colony-stimulating factor (G-CSF).
Recommendations
At IMS, the findings of a systematic review of 9 studies evaluating whether bsAbs are effective in managing extramedullary disease (EMD) and high-risk cytogenetic abnormalities (HRCAs) in RRMM—including ORRs of the entire cohort (N=660)—were reported. The ORRs for EMD and HRCAs were reported in 3 (n=78) and 4 (n=100) studies, respectively. From the studies that reported ORR for EMD, talquetamab (GPRC5D×CD3) was shown to have the highest ORR (0.45 [0.17; 0.77]), followed by elranatamab (BCMA×CD3; 0.38 [0.23; 0.55]) and teclistamab (0.36 [0.19; 0.56]). There was no significant difference in ORR among the agents with respect to EMD status. In studies that reported ORR for HRCAs, talquetamab had an ORR of 0.67 (0.17; 0.77) followed by teclistamab (0.61 [0.43; 0.76]), and elranatamab 0.55 (0.36; 0.73). Similarly, there was no significant difference in the ORR among these agents with respect to HRCA status. According to the researchers, EMD responses are significantly lower than the full cohort ORR; however, it is encouraging that responses to high-risk MM closely approximate ORR of these agents. The authors note that the reporting of EMD responses “needs to be improved, and clinical trials should report EMD responses distinctly, as it directly informs clinical decisions.”
Teclistamab, a bsAb recently approved for RRMM patients who have undergone ≥4 lines of therapy, has shown promise in clinical settings. Researchers from the Dana-Farber Cancer Institute/Brigham and Women’s Hospital reported their experience with teclistamab in 34 patients (median age 65; median 6 prior lines of therapy). Notably, 62% of patients had HRCAs, and 38% had EMD. Observed hematologic toxicities included anemia, neutropenia, and thrombocytopenia, and cytokine release syndrome (CRS), primarily grade 1 or 2, occurred in 56% of patients. Neurological toxicity was minimal, affecting 3% of patients. Infectious complications were noted in 32% of cases, with 9% classified as grade 3 or higher and no treatment-related deaths recorded. At a median follow-up of 6 weeks, ORR was 44%, with 9% achieving CR and 29% reaching VGPR or better. Teclistamab also demonstrated efficacy in patients with renal dysfunction, EMD, and/or HRCAs. According to the researchers, “teclistamab treatment in a commercial setting generated comparable responses to the previously reported clinical trials without any new toxicity signals.”
Forimtamig, a GPRC5D×CD3 T cell–engaging bsAb, has demonstrated significant clinical efficacy across various high-risk subgroups of RRMM patients, according to findings from a phase 1a dose-escalation study. In this first-in-human study, which included patients who were heavily pretreated and refractory to both proteasome inhibitors and immunomodulatory drugs, forimtamig exhibited an ORR of 66.7%, with 54.2% of patients achieving a VGPR or better. Importantly, the median duration of response was 12.2 months, with a majority of responders maintaining their responses at the time of data cutoff. Researchers also saw promising results in specific high-risk subgroups, including patients aged ≥65, those with >4 prior lines of therapy, and individuals with HRCAs. Of note, patients with 1q21 gain, known to be a HRCA, exhibited an ORR of 86.7%. Forimtamig also demonstrated effectiveness in patients previously treated with BCMA-targeted therapies, including antibody-drug conjugates, bsAbs, and CAR T-cell therapy, suggesting its potential as a salvage therapy. The study’s authors emphasized the need for further optimization of forimtamig dosing and scheduling and ongoing evaluation of its long-term treatment benefits, particularly in patients with high-risk disease characteristics.
The CARTITUDE-4 trial is a global, open-label, randomized controlled trial comparing ciltacabtagene autoleucel (cilta-cel) with physician’s choice of standard of care (SOC): either pomalidomide, bortezomib, and dexamethasone (PVd) or daratumumab, pomalidomide, and dexamethasone (DPd) in lenalidomide-refractory MM patients. As of November 1, 2022, median follow-up was 15.9 months (range, 0.1–27), and 208 patients were randomized to cilta-cel (of whom 176 received cilta-cel) and 211 to SOC. Cilta-cel significantly improved PFS compared to SOC (median not reached vs 11.8 months), with a hazard ratio (HR) of 0.26 (P< 0.0001). In prespecified subgroup analyses, cilta-cel consistently demonstrated improved PFS across various patient subgroups. This includes patients <65 and 65–75 years of age and those who have any of the following: HRCAs, ≥1 HRCAs, ISS stage III, soft tissue plasmacytomas, high bone marrow plasma cell counts, or triple-class–refractory disease. Cilta-cel also showed efficacy when compared to both PVd and DPd. According to the researchers, the benefit shown was “similar to that seen in the overall ITT population, confirming efficacy of a single cilta-cel infusion in a range of clinically relevant MM subgroups.”
Autologous GPRC5D CAR T-cell therapy shows promising results in RRMM patients who have previously undergone anti-BCMA CAR T-cell therapy, according to the latest findings from a phase 2 trial. In this single-arm study conducted in China, 11 patients with RRMM who had previously received anti-BCMA CAR T-cell therapy were enrolled and treated. At a median follow-up of 14.8 months, all 11 patients achieved a response, with 45% achieving a CR or better. Of note, 73% of patients were MRD negative in bone marrow. The median PFS was 6.4 months, and 45% of patients remained progression-free at the time of analysis. The safety profile of GPRC5D-targeted–CAR T-cell therapy was manageable, with grade 3 or higher hematological toxicities being the most common AEs. CRS occurred in 91% of patients, but all cases were grade 1 or 2, and CRS was effectively managed with tocilizumab and dexamethasone. No neurological toxic effects were reported. According to the researchers, “GPRC5D-targeted CAR T-cell therapy is clinically active with a favorable safety profile in patients who do not respond to or relapse after anti-BCMA CAR T-cell therapy.”
PHE885, a fully human CAR T-cell agent manufactured using the T-Charge platform, has demonstrated durably persistent CAR T expansion in a phase 1 study conducted at the Dana-Farber Cancer Institute. Though CAR T-cell therapy targeting BCMA has shown benefit in the RRMM setting, challenges such as lengthy manufacturing times and limited in vivo persistence still need to be resolved. PHE885 had previously demonstrated a 98% ORR across all dose levels, with a 100% ORR at doses exceeding 5×106 CAR T cells. The latest analysis of serial samples from 32 patients suggested that the manufacturing process successfully preserved stem-like memory T cells in the final product, resulting in a diverse and proliferative CAR T expansion following infusion. CAR T cells also maintained a diverse T-cell receptor repertoire, particularly in patients with long-term persistence. The researchers concluded that “T-Charge… successfully preserved stem-like memory T cell clones in the final product, leading to a highly heterogeneous and proliferative CAR T expansion with durable persistence.”
A fourth-generation BCMA CAR T-cell therapy, InstanCART, has exhibited promising results in the treatment of RRMM during a phase 1 clinical trial. Typically, CAR T-cell therapy has prolonged production times and high costs. The traditional production process, which the China-based researchers have called TraditionCART, takes 9–14 days, leading to extended vein-to-vein times and disease progression during production. By contrast, InstanCART, manufactured using the Instant Manufacturing Platform, offers a streamlined production process, optimizing T cell function. The latest phase 1 clinical trial compared the safety and efficacy of TraditionCART and InstanCART in RRMM patients. Both approaches demonstrated favorable safety profiles, with no grade 3 or greater neurotoxicity or CRS observed. However, InstanCART showed lower rates of grade 3 AEs than did TraditionCART. Notably, InstanCART achieved an ORR of 100%. There was no statistically significant difference in PFS and OS between InstanCART and TraditionCART. However, the expansion and duration of InstanCART cells was significantly higher than TraditionCART cells, highlighting its potential for enhanced therapeutic benefit. According to the researchers, InstanCART was well tolerated and showed noninferior efficacy and more encouraging pharmacokinetic profile than TraditionCART for RRMM therapy. The study is ongoing, and long-term follow-up will assess durable efficacies.
——————————————————
Jointly provided by the MMRF and RedMedEd.
This educational activity is supported by educational grants from AbbVie Inc., Bristol Myers Squibb, and GSK, and sponsorship from Legend Biotech USA Inc.
Is there a suggested sequence for the use of CAR T cells and bispecific antibodies?
There are two main challenges with finding the most appropriate sequence of CAR T cell therapy and bispecific antibodies. First, treatment sequencing has not been clinically addressed adequately and second, patients may not always receive the exact same sequence. Many clinicians will opt to offer CAR T cell therapy first for their relapsed/refractory patients due to the impressive response rates and duration of response. Furthermore, since CAR T cells are a “one-and-done” treatment, the time that patients get to experience off therapy allows their bodies and immune system to recover. However, CAR T-cell therapy can take several weeks to prepare which makes a patient’s disease biology an important factor to consider when deciding between a CAR T cell or a bispecific antibody. For example, patients for whom waiting a few weeks is not possible because their myeloma is aggressive (that is, fast-growing) might be better candidates for a bispecific antibody than a CAR T cell. But if the myeloma is slow-growing and there is a CAR T slot available, CAR T cell therapy would be the best choice.
However, patients shouldn’t get discouraged if they receive a bispecific antibody before CAR T cell therapy. Clinical trial data suggests that patients with relapsed/refractory multiple myeloma who are treated with CAR T therapy after bispecific antibodies can have an effective response. Furthermore, there are other types of CAR T cells under investigation that have different targets. Ultimately, bispecific antibodies are good options.
Do bispecific antibodies work in patients with high-risk multiple myeloma?
About half the patients in clinical studies of CAR T cells and bispecifics are considered high risk based on standard cytogenetic definitions like chromosomal translocation 4;14 or chromosome 17p deletion, among others. And in spite of having these cytogenetic abnormalities, patients are responding to these treatments; therefore, having high risk cytogenetics may not impact outcome as much with these types of treatments. However, most of these observations have come from single-arm studies and only a randomized, phase 3 study will help to discern whether any treatment is preferentially benefitting a high-risk subgroup of patients (for example, a large group of patients that are randomized to treatment A versus treatment B, and standard-risk and high-risk patients in each group are compared).
One type of high-risk myeloma patient that tends not to do well on bispecific antibody therapy, based on clinical study data, are those with extramedullary disease (EMD; that is, myeloma cells that are growing outside of the bone marrow). Patients with EMD do respond to treatment, but their response rate is not as high and not as long-lasting as in patients without EMD. Other strategies, such as combination therapies, are needed for these patients.